Delivery of Cationic Antimicrobials to Bacterial Infections Using Polyelectrolyte Surfactant Nanoparticles
Speaker:
Charles M. Roth, PhD
Professor and Chair, Department of Biomedical Engineering
Rutgers University, New Brunswick, NJ
Abstract:
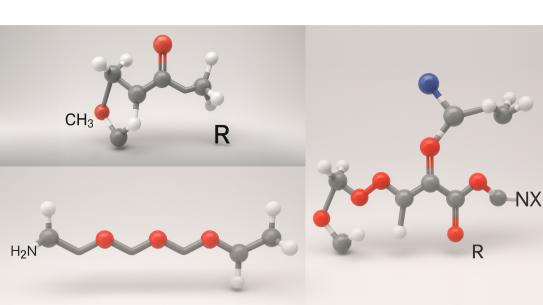
Persistent lung infections with Pseudomonas aeruginosa are a major cause of morbidity and mortality in cystic fibrosis patients. These infections are difficult to treat because thickened mucus and biofilm formation hinder the penetration and activity of standard cationic antimicrobials such as tobramycin and polymyxin B. To address this challenge, Prof. Charles Roth and colleagues have developed a new class of polyelectrolyte surfactant nanoparticles designed to encapsulate and deliver cationic drugs more effectively. These nanoparticles are created from graft copolymers consisting of an anionic poly(alkylacrylic acid) backbone with polyetheramine side chains, which self-assemble with cationic antimicrobials through electrostatic interactions. By tuning backbone chemistry and graft density, the researchers were able to control particle size, drug binding strength, and release kinetics. When tested against clinical isolates of P. aeruginosa, these formulations showed significantly enhanced antimicrobial activity against planktonic bacterial cultures, particularly with the PMAA-g-10%J variant, while maintaining stability over time. Against established biofilms, the nanoparticles generally retained, but did not surpass, the activity of free drugs—though strain-specific improvements were observed. Mechanistic studies revealed that the nanoparticles interact directly with bacterial membranes and alter depolarization responses, suggesting a mode of action that enhances drug delivery to cells. This work provides a proof-of-concept for nanoparticle-enabled delivery of cationic antimicrobials and highlights the potential of molecular engineering strategies to overcome the biological barriers that make chronic lung infections so difficult to treat.
Dr. Roth, earned his B.S.E. in Chemical Engineering from the University of Pennsylvania (1989) and his Ph.D. in Chemical Engineering from the University of Delaware (1994), followed by postdoctoral training in Bioengineering at Harvard Medical School and Massachusetts General Hospital (1995–1997). Before joining Rutgers, he served as Instructor in Surgery and Bioengineering at Harvard Medical School. Since 2000, he has been on the Rutgers faculty, where he has directed a research program in nanomedicine and molecular systems bioengineering with applications to cancer, infection, and drug delivery. He is a Fellow of the American Institute for Medical and Biological Engineering (AIMBE) and recipient of numerous honors including the NSF CAREER Award, the Whitaker Foundation Transitional Career Award, and Rutgers’ Warren I. Susman Award for Excellence in Teaching.