Controlling the local reaction environment to drive catalysis

Speaker
Max Huelsey
Massachusetts Institute of Technology
Massachusetts, USA
Abstract
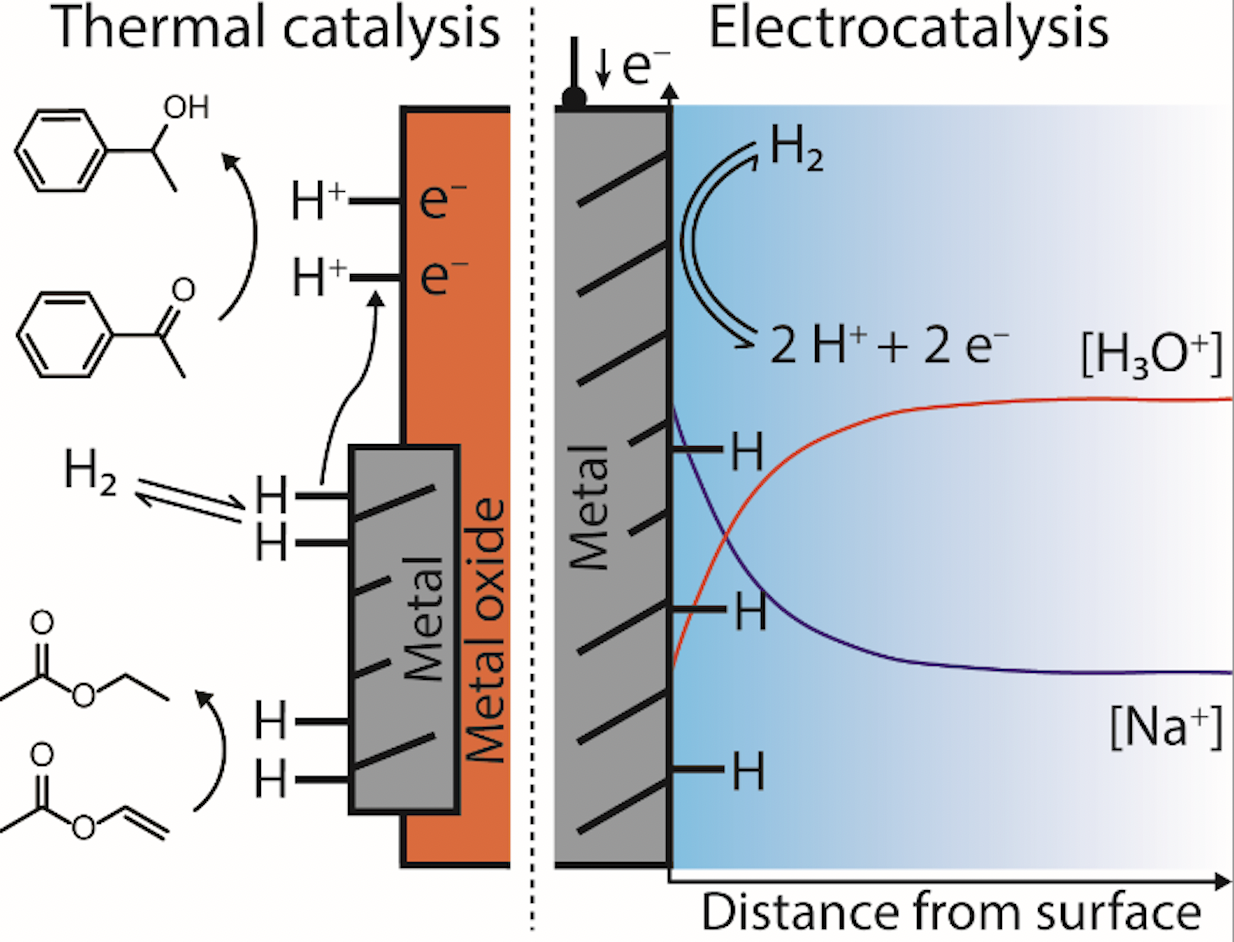
Understanding and driving changes in the local environment around catalytic active sites is crucial for optimizing their activity. I will describe how ion transport determines the reaction environment and catalyst structure both in thermal and electrocatalytic reactions. Under cathodic electrolysis, the constant consumption of H+ equivalents can lead to drastic changes in the local pH environment, an effect that is predominantly driven by the transport competition of hydronium and supporting electrolyte cations. Ion exchange polymer overlayers determine the local pH environment and thus have a direct impact on the relative CO2 reduction/hydrogen evolution efficiency. Although ion transport is less commonly invoked to be relevant in thermal catalytic reactions, hydrogen spillover – that is the transport of ‘H atoms’ from metallic sites to a reducible support – is a commonly observed phenomenon. Through experiments on a model catalyst system, we revealed how the transfer of protons and electrons to a reducible support affect the intrinsic hydrogenation activity of catalytic active sites for a variety of reducible compounds.
Bio
After having finished undergraduate and master’s degrees in biochemistry and chemistry, respectively, at Heidelberg University (Germany), Max received his PhD degree in chemical engineering from the National University of Singapore under the tutelage of Prof. Ning Yan. Currently, he is a Schmidt Science Fellow at the Massachusetts Institute of Technology in the group of Yogesh (Yogi) Surendranath.

