Using Light Energy to Accelerate Electrochemical Reactions in Li-ion Batteries

Speaker:
Chris Johnson, Ph.D.
Argonne National Laboratory, Chemical Sciences and Engineering Division
Chris Johnson is currently an Argonne Distinguished Fellow and senior chemist at Argonne National Laboratory, specializing in the research & development of battery materials and battery systems with 30 years of experience. He is known worldwide for his development of state-of-art lithium-ion battery cathode materials, and sodium-ion batteries. Recently he has been interested in accelerating electrochemical reactions with light. He holds a BS. Chemistry from the University of North Carolina at Chapel Hill and a Ph.D. in Chemistry from Northwestern University. He has published over 134 publications, and 34 issued US patents in the battery field. He has received the research award from the International Battery Association in 2006, and a R&D-100 award for the commercialization of lithium battery materials in 2009. He is Past-Chair of the Electrochemical Society Battery Division, and immediate past President of the International Battery Association (IBA). He is the 2018 recipient of the University of Chicago Argonne Distinguished Scientist Award, and is a Fellow of the Electrochemical Society.
Abstract:
The need for energy storage and its rising demand has become a major issue that the world faces today and going forward in the future. Lithium-ion (Li-ion) batteries are widely used for energy storage in a myriad of portable consumer applications and now are being employed heavily for transportation (Electric vehicles or EVs) technologies. High volumetric energy density and low weight of the batteries have enabled EVs to gain market share. The specific energy and power of Li-ion batteries continues to grow as high-performance anode and cathode materials become commercially available; however fast-charging of EV batteries still remains a challenge. This presentation will focus on accelerating electrochemical reactions, particularly for fast charging Li-ion batteries using light energy processes. An example of a ‘window’ coin-cell is shown below that represents how light propagates onto an electrode material to do the interfacial photochemistry, in this case on Li4Ti5O12 spinel anode. So-called photo-assisted fast charging employed on the electrode materials in the battery pack can alleviate concerns about slow charging that hampers the consumer acceptance and ‘range anxiety’ of EVs.
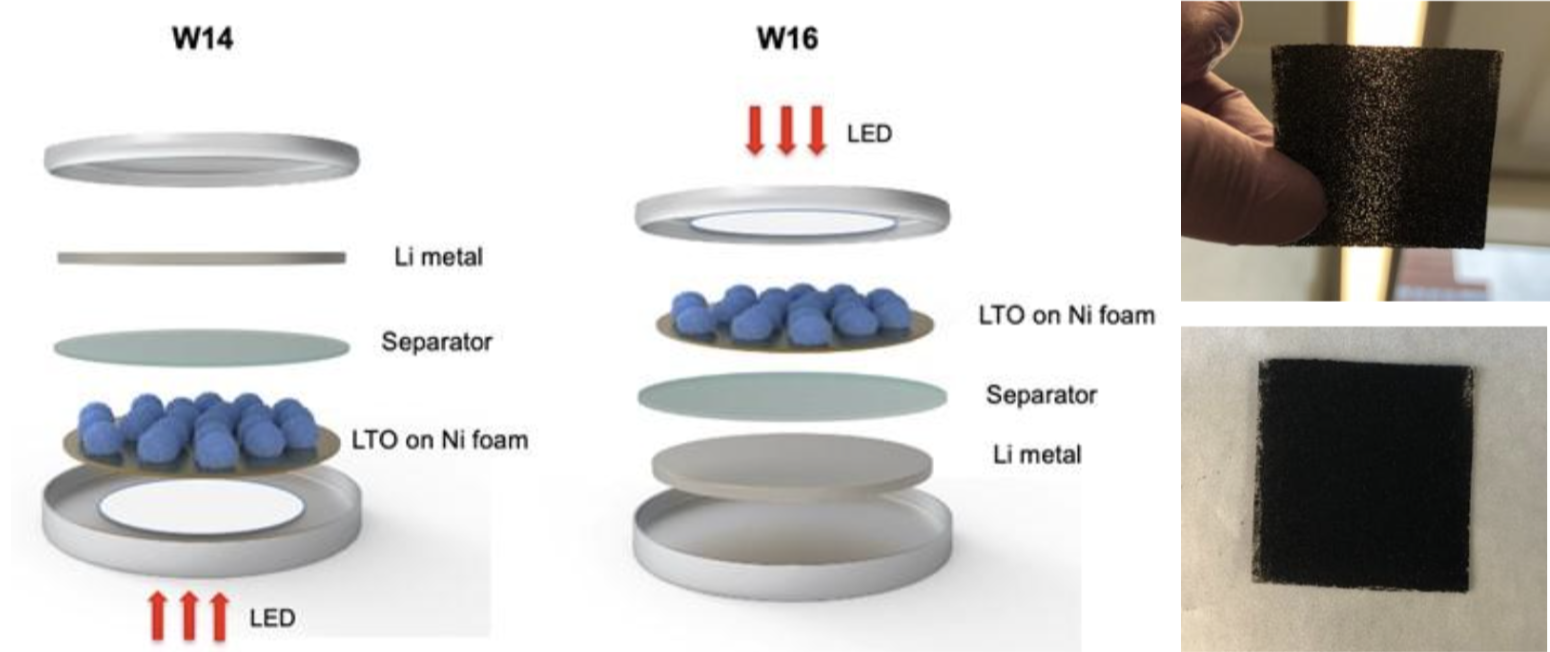
Window cell configurations with Li4Ti5O12 (LTO) anode on Ni foam electrodes. Digital photos of LTO coated Ni foam.
The submitted manuscript has been created by UChicago Argonne, LLC, Operator of Argonne National Laboratory (“Argonne”). Argonne, a U.S. Department of Energy Office of Science laboratory, is operated under Contract No. DE-AC02-06CH11357. The U.S. Government retains for itself, and others acting on its behalf, a paid-up, nonexclusive, irrevocable worldwide license in said article to reproduce, prepare derivative works, distribute copies to the public, and perform publicly and display publicly, by or on behalf of the Government.

