Establishing Compositional Control in 2D and 3D Metal Sulfide Electrocatalysts to Drive CO2 and CO Conversion to Alcohols
Speaker:
Jesús M. Velázquez
UC Davis, Department of Chemistry
Abstract:
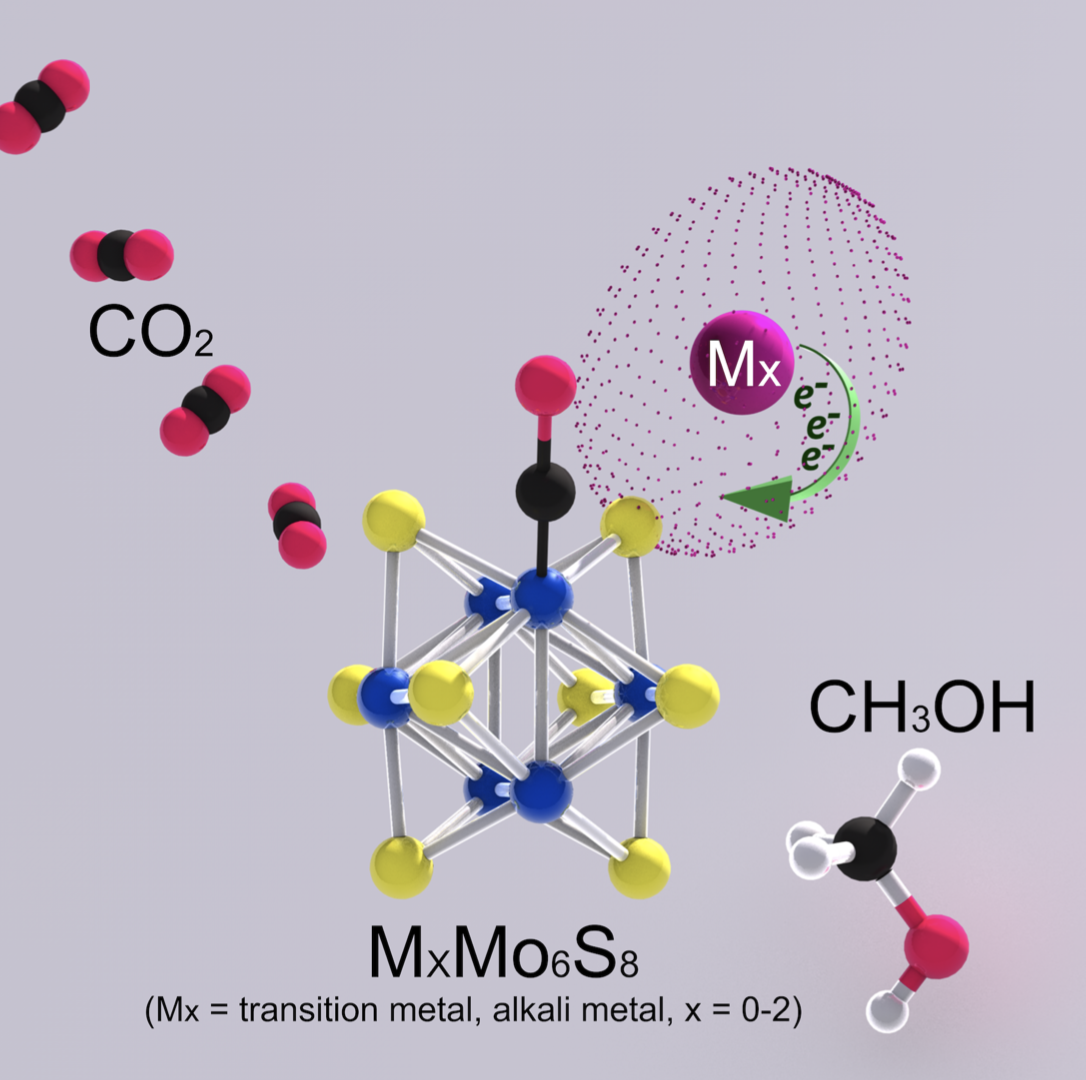
Development of materials that address the growing dichotomy of simultaneously increasing energy demands and carbon emissions is an imperative that has progressively affected energy-related research ef- forts. An emerging technical avenue in this area is the conversion of vastly abundant renewable energy sources that can be harnessed and directed towards the synthesis of traditionally fossil fuel-based products from atmospheric feedstocks like CO2. To this end, our work establishes structure-function relationships for materials within the versatile classes of MX2 (M = Mo, W; X = S, Se) and Chevrel-Phase (CP) MyMo6X8 (M = alkali, alkaline, transition or post-transition metal; y = 0-4; X = S, Se,Te) chalcogenides. The molybdenum sulfide structures from both families exhibit exceptional promise as CO2R catalysts. Furthermore, we have identified the CP catalyst framework as being selective towards the electrochemical reduction of CO2 and CO to methanol (only major liquid-phase product) under applied potentials as mild as -0.4 V vs RHE. Reactivity toward electrochemical reduction of CO2 and CO to methanol is correlated with increased population of chalcogen states, as confirmed via X-Ray Absorption Spectroscopy. Overall, this work seeks to unravel optimally reactive novel small-molecule reduction catalyst compositions.