Diversity and Design: New Chemical Probes for Biology and Medicine
Speaker:
Derek S. Tan
Memorial Sloan Kettering Cancer Center
Abstract:

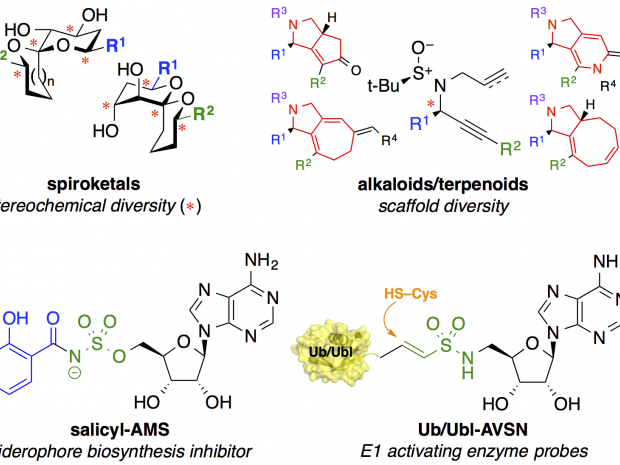
Existing small-molecule drugs address only a small set of ≈550 protein targets that are encoded in the human genome. Efforts to expand beyond this narrow range of biological targets have been thwarted by a heavy focus on a correspondingly narrow range of complementary chemical structures in modern drug discovery. To address this problem, we leverage insights from natural products in both diversity-oriented synthesis and rational drug design to identify novel small-molecule ligands for a variety of targets in infectious diseases and cancer. In the area of diversity-oriented synthesis, we use structural motifs found in biologically active natural products as attractive starting points for library design. At the core of these efforts is the development of new synthetic routes to provide flexible, efficient, systematic access to these structures. Along these lines, we have developed syntheses of spiroketals, polyketides, alkaloids, macrocycles, medium rings, and oxygen heterocycles having stereochemical and skeletal diversity. These libraries access distinct regions of chemical space compared to conventional drug-like libraries and are being screened against a wide range of biological targets. In the area of rational drug design, we are engaged in complementary efforts to design natural product-based sulfonyladenosine inhibitors of adenylation enzymes, a mechanistic superfamily that is implicated in a wide range of biological processes and includes promising new antibacterial and anticancer targets. Leveraging structural and mechanistic information about individual targets of interest, we have developed new antibiotic lead compounds that inhibit biosynthetic enzymes required for virulence of pathogenic bacteria, including Mycobacterium tuberculosis. In related work, we are developing a general platform to assess small-molecule permeability in bacteria and correlations with physicochemical properties in an effort to address this major obstacle in antibiotic drug development. We have also designed semisynthetic protein inhibitors of enzymes in the ubiquitin conjugation cascade to probe active site remodeling during catalysis. We leverage multidisciplinary collaborations with biologists to evaluate the molecules we synthesize with the long-term goals of probing complex biological processes and pursuing new therapeutic opportunities in cancer and infectious diseases.
- 10:30 Refreshments
- 10:45–12:00 Talk

