Researchers at NYU Tandon Create New Biomaterial that Delivers Both a Powerful Drug and Gene Silencers
New hybrid shows promise in dealing double blow to cancer cells by delivering both a chemotherapeutic agent and RNA Interfering Technology that Silences Drug Resistance
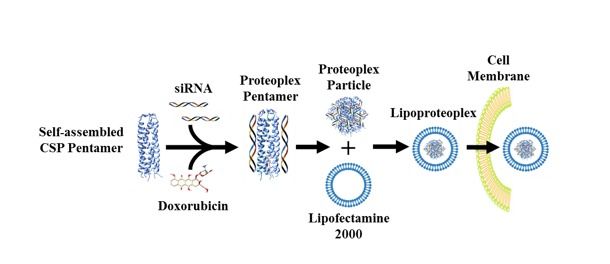
Lipoproteoplex uses a lipid “container” for transfection — the transportation of material past a cell membrane — and an easy-to-make protein capsule that can bind both small-molecule chemotherapeutic drugs and genetic technology, such as short interfering RNA (siRNA), that can "silence" genes that propagate disease states.
BROOKLYN, New York – Clinicians today have an arsenal of more than 200 drugs at their disposal for treating a range of cancers — 68 drugs were approved between 2011 and 2016 alone. But many chemotherapeutic agents pose stubborn challenges: they cause serious side effects because they kill healthy cells in addition to cancer cells; some forms of cancer develop resistance to drugs; and many such chemotherapies, being poorly water-soluble, demonstrate low bio-availability resulting in sub-optimal drug delivery to cancer cells.
A potential solution lies in the synergistic combination of a chemotherapeutic drug with engineered genetic material designed to neutralize the malevolent genes conferring resistance to that drug, among other functions.
While there are numerous examples of synthetic dual gene and drug delivery vehicles, new hybrid materials developed in the lab at the NYU Tandon School of Engineering use easily modifiable proteins to deliver a chemical one-two punch: they combine a lipid “container” for transfection — the transportation of cargo past a cell membrane — and an easy-to-make protein capsule that can bind both small chemotherapeutic molecules and nucleic acids.
Developed by a team led by NYU Tandon Associate Professor of Chemical and Biomolecular Engineering Jin Kim Montclare — who also serves as an Affiliate Professor of Chemistry at NYU’s College of Arts and Sciences, and an Affiliate Professor of Biomaterials at NYU College of Dentistry, as well as being affiliated with SUNY Downstate as a Professor of Biochemistry — the hybrid lipid-protein material, called a lipoproteoplex, comprises both a coiled supercharged protein macromolecule and a commercially available transfection agent called Lipofectamine 2000.
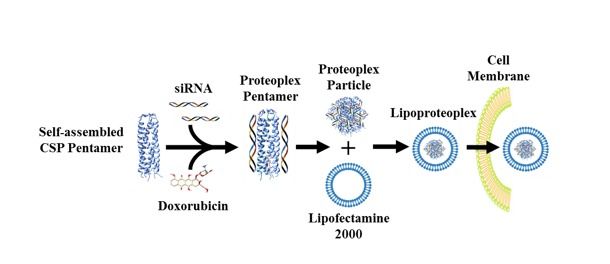
Lipoproteoplex allows researchers to swap out a supercharged protein or lipid component and any number of siRNA to address a specific cell line and type of drug.
Because the researchers engineered the protein macromolecule with extensive positive charges on the surface and a hydrophobic core, it can be easily festooned with negatively charged short interfering RNA (siRNA) — a powerful tool for suppressing genes that invoke drug resistance and propagate disease states —while also serving as an efficient, and toxicity reducing, carryall for the hydrophobic chemotherapeutic agent doxorubicin.
In research published in Biomacromolecules, a journal of the American Chemical Society, the team details how the lipoproteoplex exposed to samples of the MCF-7 breast cancer cell line delivered more doxorubicin to target cells than did Lipofectamine 2000 alone, resulting in a substantial decrease in MCF-7 cell viability. They also demonstrated that the hybrid macromolecule was highly successful at siRNA transfection, silencing the gene by 60 percent.
Montclare said a key benefit of the new lipoproteoplex is ease of modification, an asset to researchers studying cells whose genetically invoked behavior changes over time and differs by cell line and patient. Rather like a system of mix-and-match components, the lipoproteoplex allows researchers to swap out a supercharged protein or lipid component and any number of siRNA to address a specific cell line and type of drug.
“Unlike other pursuits at producing dual gene and drug delivery systems, this approach doesn't require tedious chemical synthesis procedures; rather we can biosynthesize any variant of the supercharged protein,” she said. “This allows for substituting different siRNA molecules and chemotherapeutic drugs to suit lab needs.”
In recent work on protein-based hybrid materials, Montclare and her collaborators combined an engineered supercharged protein with the transfection reagent Fugene. The combination exhibited an eightfold improvement in transfection efficiency of DNA compared to Fugene alone, with negligible cytotoxicity.
Montclare is investigating the mechanisms that enable these lipoproteoplexes to effectively deliver genes and drugs across different cell lines.
The team comprised NYU Tandon Department of Chemical and Biomolecular Engineering graduate students Raymond Chen, Joseph Frezzo, Lindsay K. Hill (also an MD candidate at SUNY Downstate School of Medicine), Che Fu Liu, Haresh T. More, Nikita Srivastava, Liming Yin, post-doctoral researcher Priya Katyal; and researchers from the NYU College of Dentistry Department of Biomaterials, NYU Department of Chemistry, NYU Center for Genomics and Systems Biology, and NYU Courant Institute of Mathematical Sciences, as well as the State University of New York Downstate Medical Center and the Simons Foundation’s Flatiron Institute Center for Computational Biology.
“Efficient Dual siRNA and Drug Delivery Using Engineered Lipoproteoplexes” is available at pubs.acs.org.
Note: Images available at http://dam.engineering.nyu.edu/?c=1975&k=28d9ea9da2
About the New York University Tandon School of Engineering
The NYU Tandon School of Engineering dates to 1854, the founding date for both the New York University School of Civil Engineering and Architecture and the Brooklyn Collegiate and Polytechnic Institute (widely known as Brooklyn Poly). A January 2014 merger created a comprehensive school of education and research in engineering and applied sciences, rooted in a tradition of invention and entrepreneurship and dedicated to furthering technology in service to society. In addition to its main location in Brooklyn, NYU Tandon collaborates with other schools within NYU, the country’s largest private research university, and is closely connected to engineering programs at NYU Abu Dhabi and NYU Shanghai. It operates Future Labs focused on start-up businesses in downtown Manhattan and Brooklyn and an award-winning online graduate program. For more information, visit engineering.nyu.edu.




