Smart polymers perform nano-acrobatics
Researchers are finding remarkable ways in which bioengineered paired macromolecules can be made to self-assemble, disassemble, and more — and then biodegrade when they’ve finished their work.
The key to these macromolecules — called block copolymers — is their ability to self-assemble when exposed to discrete external stimuli. Self-assembly can occur as a function of temperature or pH, for example. And it is not necessarily a permanent change; it can be reversed.
Genetically engineered copolymers have applications in everything from artificial therapeutics, biocatalysts, scaffolds, and cells for medicine, to sustainable energy and environmental remediation.
For four years, Jin Kim Montclare and researchers at the Polytechnic Institute of New York University have been developing block copolymers from scratch using recombinant DNA and putting them through biochemical hoops.
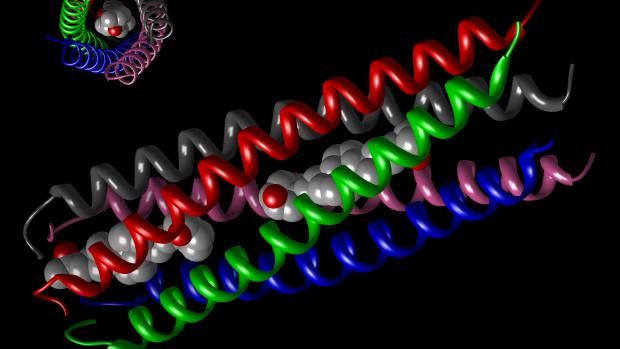
The group’s work, published recently in the journal ChemBioChem, involves block copolymers comprising elastin alternating with COMPcc. The former is a pentapeptide whose amino-acid constituents can assemble into a beta spiral structure as a function of temperature, pH, or salinity.
COMPcc, which stands for “cartilage oligomeric matrix protein coil coiled,” is a pentamer arranged as five helixes that can contort into an arrangement that produces a hydrophobic core the way one might create a cylindrical cavity by stacking garden hoses on a deck—thus the odd “coiled coil” nomenclature.
COMPcc has the ability to bind small water-insoluble molecules such as Vitamin D within its hydrophobic core. The possibilities are manifold.
“That central pore can potentially bind chemicals that are hard to deliver as drugs because they are normally not water soluble,” says Montclare.
For example COMPcc can bind to Vitamin D, a non-dissolving molecule that happens to have profound implications for regenerative tissue and serves as a signaling hormone for the promotion of tissue differentiation into cartilage and bone. And COMPcc can “live” in a copolymer with elastin, synthetics, or other coiled coil-based materials that self-assemble into gels or more organized forms like scaffolding, which can be used for tissue regeneration.
“The materials that we make form nano-scale self-assembled, biodegradable particles,” says Montclare. “So you can envision loading them with drugs and delivering them in vivo, or using them as scaffolding for tissue engineering because they are protein-based and therefore suitable for the body without risk of eliciting an immune response.”
Montclare says the advantage of using biological protein-based systems is that one has control over their three-dimensional conformation, the sequences that make up the organic blocks. “Sometimes with synthetics you don’t have that,” she says.
Montclare’s team also discovered that the self-assembling behavior of their block copolymers isn’t determined merely by the copolymers’ constituents. It is also a function of the order in which the blocks are arranged within a copolymer, and the number of times a given block appears.
Montclare says that this discovery was a big surprise.
“We were expecting there would be no difference because in synthetic block polymers there isn’t,” she says. “But with protein-derived macromolecules, we found that directionality controls assembly. . . . In our particular case, we can control the temperature-dependent self-assembly by changing the block orientation and number of blocks both on the micro and macro scale.”
Next for the lab, Montclare says, is work controlling the self-assembly of macromolecules by changing the length of each domain.
The work was funded by the Air Force Office of Scientific Research and the National Science Foundation (NSF)-supported Materials Research Science and Engineering Centers grant.




