Research Overview
Our research aims to develop microengineered translational technologies for emerging biomedical problems by micro-engineering cell behaviors and environments, and ultamately make a real impact on improving human health. Numerous revolutionary topics exist at the interface between biomedical science and micro-/nano-technology. To bridge these fields, our research is mainly focusing on translational researches that leveraging the engineering advances in biomaterials, microfluidics, and biomanufacturing for new and improved clinical solutions to emerging problems in cancer biology, immune engineering, cell mechanobiology, and stem cell-based regenerative medicine. Specific examples include microengineered lab-on-a-chip systems for single-cell analysis, immune monitoring, and tumor microenvironment modeling for cancer diagnosis and treatment screenings. Meanwhile, we are also interested in probing cell mechanics using novel micromechanical tools to profile the evolution of subcellular forces and cytoskeleton dynamics with a one-to-one spatial registration, so as to illustrate how the dynamic cell mechanics could collectively drive and regulate the functions of a single cell in healthy and disease conditions.
Microengineering organotypic cancer microenvironment models
A detailed understanding of cancer initiation, evolution and treatment resistance is indispensable for optimal therapy. Conventional cell culture and animal models are generally used in cancer modeling and related fundamental research, yet these models are compromised in dynamic monitoring and real-time analysis and lack of biomimetic human immune system. With the heterogeneity of patient responses to new cancer therapies in clinical trials, our research has established a line of novel, integrated microfluidic-based microphysiological systems that reconstitute the dynamic in vivo pathology of diverse tumor niches for patient-specific screenings in vitro/ex vivo. With our collaborators at the NYU Langone Brain Tumor Center, we developed for the first time an organotypic, patient-specific glioblastoma (GBM) tumor microenvironment model (termed GBM-on-a-Chip) that supports 3-D microvessel angiogenesis (Integrative Biology, 2016, 8, 785-794), immunosuppressive cell and cytokine milieus, and tunable extracellular soluble (cytokines) and matrix (ECM) interactions (by modulating integrin-specific ligands on ECM-based hydrogel biomaterials) (Advanced Healthcare Materials, 2018, 1801234) to dissect and disrupt therapy resistance mechanisms in anti-angiogenic therapy (Biomaterials, 2018, 161, 164-178). To extend our research to make clinical impact, we populated our bioengineered GBM-on-a-Chip system with a cohort of molecularly distinct GBM patient tumors to stratify differences in immunosuppressive cell functions and cytotoxic T-cell responses from PD-1 checkpoint immunotherapy and enhance immunotherapy efficacy for clinical trial patients ex vivo. Although many studies have been done on solid tumor microenvironments, few studies have been reported in engineering the architectures of the liquid tumor microenvironment such as leukemia. Thus, our research has for the first time engineered a novel biomimetic B-cell acute lymphoblastic leukemia (B-ALL) model (termed Leukemia-on-a-Chip) to dissect key chemotherapy resistance mechanisms in the liquid bone marrow stromal microenvironment. Our research team is now further engineering a novel biomimetic ex vivo tumor immunity model to dissect the leukemia bone marrow immune microenvironment and validate a personalized CAR T-cell immunotherapy regimen for improving efficacy and safety in relapsed and refractory leukemia patients.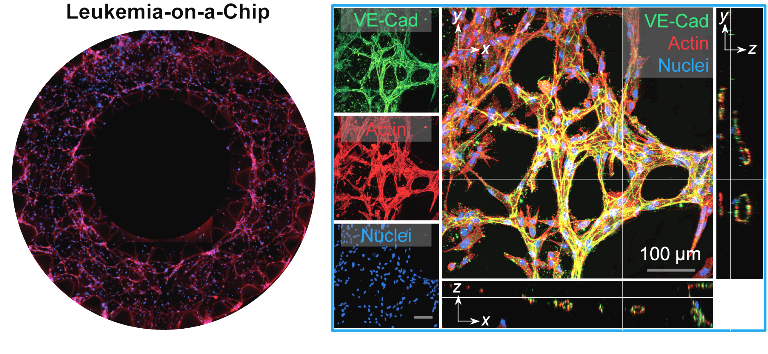
Microfluidic Biosensing Technologies for Multiplex, Real-time Immunophenotyping
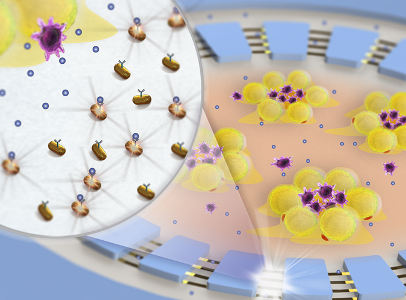
An accurate and real-time measurement of immune cell functions for patients is critical yet challenging in disease diagnosis and prognosis and personalizing immunotherapy regimens. Particularly, a quantitative analysis of the dynamic cytokine secretion profiles of immune cell subsets are required for a precise determination and characterization of the immunophenotype of patients. Therefore, our research contributed a new integrated microbead-based microfluidic platform for efficient isolation, enrichment, stimulation, and multiplexed cytokine secretion profiling of diverse immune cells from whole blood (Lab on a Chip, 2018, 18, 522-531; Biomicrofluidics, 2016, 10, 054114). This allows for systemic-level immunophenotyping to define and characterize the immune status of healthy individuals and diseased patients, critical for deciding clinical interventions and management of patients with immune disorders.Despite many advanced biosensing techniques have been purposed for cytokine profiling, there are no clinically available methods that integrate high-resolution immune cell monitoring and label-free, multiplexed cytokine detection together in a biomimetic tissue microenvironment. Recently, we developed a localized surface plasmon resonance (LSPR)-based nanoplasmonic barcode-based biosensor for a rapid, multiplexed, label-free, and in situ cytokine analysis (Advanced Healthcare Materials, 2019, 8, 1801478), and demonstrated for the first time an integrated inflammatory adipose tissue-on-a-chip system to monitor in situ cytokine profiles on-chip for 15 days in a biomimetic tissue microenvironment under different therapies (Lab on a Chip, 2018, 18, 3550-3560). In collaboration with Drs. Pengyu Chen (Auburn University) and Matija Snuderl (NYU School of Medicine), we will further develop a novel, integrated nanoplasmonic biosensor that allows direct visualization and mapping of cytokine production, diffusion, transportation to better dissect the functional diversity of immune cell crosstalk in glioblastoma brain cancer immunotherapy. To extend this research to the clinical setting, we will implement our microfluidic nanoplasmonic biosensor to further provide detailed, time-dependent mechanisms of patient-specific CAR T-cell responses, at a single cell resolution, during stimulation and evolution in a 3-D immunosuppressive tumor niche for preclinically screening of optimal, effective and safe CAR T-cell therapy. Our research on the nanoplasmonic microfluidics-based biosensing platform would provide a vital signature that quantitatively characterizes the immunophenotypes of patients and would therefore allow for a more precise diagnosis and stratification of cancer and immune diseases.
Capture and characterization of rare circulating tumor cells
Circulating tumor cells (CTCs) detached from both primary and metastatic lesions represent a potential alternative to invasive biopsies as a source of tumor tissue for the detection, characterization and monitoring of cancers. Our early publications demonstrated nanotopographical materials can be integrated onto a microfluidic platform for efficient and non-invasive capture of rare, viable circulating tumor cells (CTCs) from the blood of breast cancer patients for detecting, characterizing, and monitoring tumor metastasis (BMC Cancer, 2016, 16, 614; ACS Nano, 7, 566, 2013). Our method uniquely utilized the differential adhesion preference of cancer cells to nanoroughened surfaces, as compared to blood cells, and, thus, did not depend on their physical size or surface protein expression, a significant advantage unlike existing CTC capture techniques. We further extended this pioneered research to study the timing of CTC release from primary prostate tumors, the heterogeneity in molecular/cellular properties, and representativeness of either primary or metastatic tumor deposits for an integrated and informative analysis to predict patient prognosis.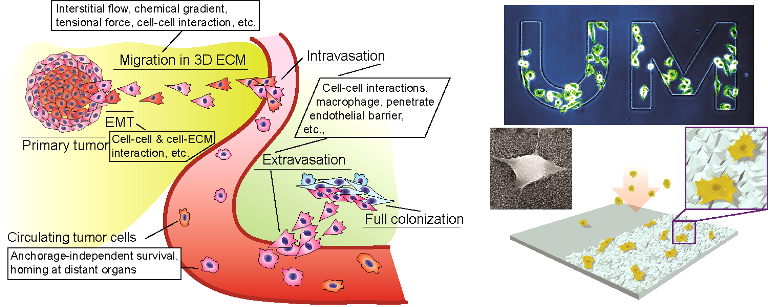
Cancer mechanobiology
Studies have correlated the malignant phenotype, metastatic potential, and tissue tropism of cancer cells to their mechanical properties (i.e. adhesion, deformability, and forces). Thus, biomechanical properties of cancer cells, particularly cancer stem cells (CSCs) and CTCs, may serve as a diagnostic for cancer. To explore and validate this, my research team has developed new microfluidic and micromechanical tools for efficient cell trapping and high-throughput, highly-sensitive, and label-free quantification of cell size, deformability and expression levels of surface receptors of live single cancer cells (Small, 2016, 12, 2300-2311; Small, 2019, 1802891). In collaboration with Dr. Mohammad Qasaimeh at NYU Abu Dhabi, we applied our established microfluidic CTC chip to capture rare CTCs from prostate patients, and, subsequently, characterize the mechanophenotype of CTCs using an integrated microfluidic-atomic force microscopy (AFM) platform. Moreover, in collaboration with Dr. Matija Snuderl at NYU School of Medicine, we examined the biomechanical attributes of the tumor microenvironment that contribute to the evolution of CSCs and discovered that the imbalance between the extracellular and intercellular transmission of cell contractility defines the CSC phenotype. An accurate profiling of the mechanophenotype of cells from cancer patients will provide new insight into the origin and regulation of malignant phenotype and metastatic tumors.Stem cell mechanobiology
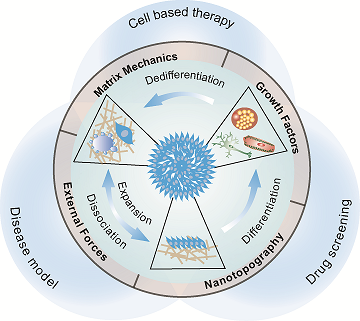
Human stem cells, including pluripotent stem cells (hPSCs), and adult stem cells, such as mesenchymal stem cells (MSCs), have a unique capacity for indefinite self-renewal in vitro coupled with their ability to differentiate into various specific cell types present in the human body, making them invaluable tools for studying tissue formation in vitro and a promising resource for regenerative medicine. Over the past two decades, stem cell biologists have assembled considerable knowledge of how biochemical factors, signaling pathways, and transcriptional networks regulate human stem cell behaviors. However, it has recently become clear that the coexisting biophysical properties in the stem cell niche, such as substrate rigidity (Nature Materials, 2014, 13, 599-604) and nanotopographic cues (Nanoscale, 2018, 10, 3556-3565; ACS Applied Materials & Interfaces, 2017, 9, 41794-41806; Advanced Healthcare Materials, 2015, 4, 1900-1914) can provide potent control over a variety of cell behaviors, including the self-renewal and differentiation properties of stem cells. Our research has been oriented towards engineering synthetic micro/nano-scale biomaterials to explore critical regulatory roles played by the dynamic biophysical signals in the local stem cell microenvironment (such as cell shape and geometry, matrix mechanics, external mechanical forces, and nanotopographical features of the ECM) that regulate the spatiotemporal adhesion-mediated signaling and downstream stem cell self-renewal and differentiation.At the cell-ECM interface, both in vivo ECM and cell membrane are enriched with adhesive molecules and structures with spatial organizations and characteristic dimensions of a few to hundreds of nanometers that regulate stem cell functions. Hence, our research aims to use bioengineered approaches to control the nanotopographical features of the local stem cell niche and develop a mechanistic understanding of the functional regulatory network involving stem cell nanotopographical sensitivity and functions, dubbed nanotopography-relayed mechanotransduction. Therefore, our publications demonstrated novel, fully defined culture systems with tunable rigidity or nano-scale surface topography to drastically improve the yield and purity of hPSC neural differentiation (Nanoscale, 2018, 10, 3556-3565). More importantly, for the first time, our studies revealed that nanotopographical cues significantly influence integrin activation and endocytosis, and thereby directly regulate interactions with the ECM and proteins involved in adhesion signaling, actomyosin, Rho GTPase, and BMP4/Smad signaling, which in turn directs hPSC function and neural lineage differentiation. Based on this study, we also developed a novel, fully defined culture system encoded with biophysical nanoscale cues to drastically improve the yield and purity of neural differentiation of hPSCs, in order to expand transplantable neural stem cells for treating stroke-related neurological disability.
Successful bone tissue engineering requires the development of implant biomaterials that can stimulate MSCs to become osteoblasts. Our research therefore explored how extracellular nanotopographic cues and YAP/TAZ mechanotransduction signaling affect MSC osteogenic differentiation for bone repair (ACS Applied Materials & Interfaces, 2017, 9, 41794-41806). Mechanotransduction in stem cells is a completely new and unexplored research territory, and my research provides innovative approaches to characterize and understand the mechano-sensing and -transduction mechanisms and its involvement in regulating self-renewal and differentiation of stem cells.
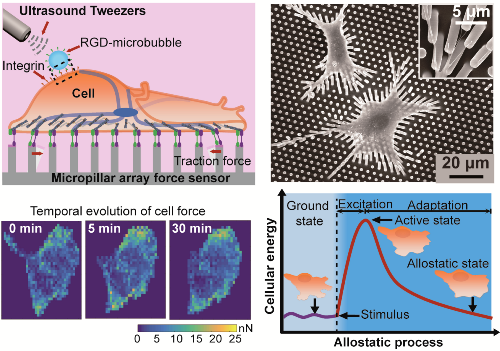
Exploring single-cell mechanical allostasis using ultrasound tweezers
Cells actively modulate mechanobiological circuitry against external perturbations to stabilize whole cell/tissue physiology through a dynamic process, termed cell mechanical allostasis. This ability to achieve physiological stability through change requires coordination of a multitude of biochemical and biomechanical dynamics in very small dimensions (nano- to micro-meters) and timescales (seconds or minutes). Dysregulation of such dynamics has been associated with pathophysiological conditions in cardiovascular disease, aging, cancer, and developmental disorders which remain hardly explored.To explore mechanotransduction in cellular allostasis, our research has developed an integrated micromechanical system that combines an ultrasound tweezers-based mechanical stressor and a Fo?rster resonance energy transfer (FRET)-based molecular force biosensor, coined actinin-sstFRET,to non-invasively monitor in situ single-cell allostasis in response to controllable, transient stimulation in real time (Cellular and Molecular Bioengineering, 2019, s12195-019-00578-z). By integrating tools that simultaneously permit localized mechanical perturbation and map actomyosin forces, we determined that cellular mechanical allostasis is dependent on a mechanosensitive bio-chemo-mechanical feedback (Ca2+ influx, CSK contraction dynamics, and GTPase RhoA signaling). In addition to the study of mechanobiological signaling, our research has also tried to explore the physics principles involved in the cellular allostasis process. Using our innovative micromechanical system, we for the first time discovered an energy-mediated machinery for driving cellular mechanical allostasis (Advanced Materials, 2019, 1900453). With the development of novel theoretical frameworks, our findings highlight the biophysical origin of cellular mechanical dynamics, providing subcellular methods to understand the etiology and progression of diseases or aging.
To extend our research to make clinical impact, we explored the biological mechanisms of cell mechanical allostasis and maladaptation in disease and try to reveal the pathogenic contexts and the biophysical mediators that underlie disease development. In collaboration with Dr. Bhama Ramkhelawonat at NYU School of Medicine and using our ultrasound tweezers systems, we performed comprehensive dynamic mechanophenotyping of single vascular smooth muscle cells to define and characterize the physiological status of healthy and diseased cells in abdominal aortic aneurysm (AAA). We for the first time use the cellular biophysical dynamics to predict the progression of AAA and potential drug efficacy in AAA. In another study, we also exploited to distinguish healthy and type II diabetic vascular smooth muscle cells (Cellular and Molecular Bioengineering, 2019, s12195-019-00578-z). We will also implement our technique to further explore detailed mechanical dynamics mechanism of how CAR T-cell executes cancer cell death to improve immunotherapy efficiency. Therefore, our research on exploring single cell mechanical allostasis with integrated micromechanical system will provide an unprecedented and innovative in vitro platform for exploring biophysical mechanisms of diseases and prediction of drug efficacy in cardiovascular disease and cancer treatments.